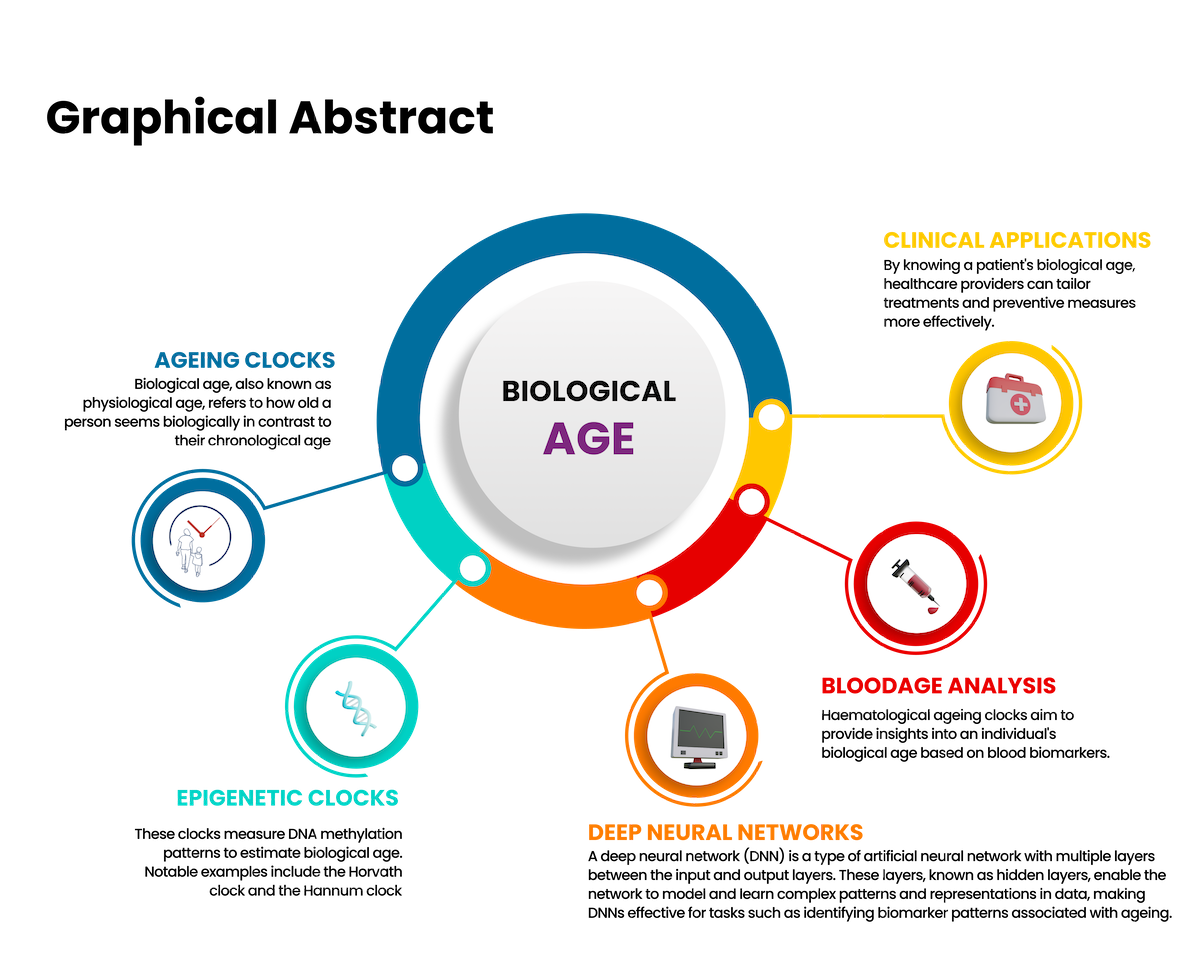
Abstract
This narrative review delves into the evolution and clinical utility of ageing clocks in predicting biological age. It highlights their development, accuracy, and application in both research and clinical settings. Results from studies underscore the importance of biological age as a more precise indicator of health and disease risk compared to chronological age. The review explores the role of ageing clocks in personalised medicine, risk assessment, and monitoring health over time. Of the various ageing clocks available, the blood age clocks provide a framework for healthcare providers to discuss various aspects of health with their patients including nutrition, exercise, lifestyle choices, medication and preventive medicine. Blood age clocks provide tangible, graphic evidence of biological aging, which can be easily understood by patients. This visual representation helps in identifying specific biomarkers that need attention, making it easier for patients to comprehend their health status and the impact of their lifestyle choices. The blood clock model is not only cost-effective, but also easy to use. It has the potential to be integrated into routine clinical practice to identify interventions that could slow down the aging process and improve the quality of life.
Keywords
Biological age, ageing clocks, blood age, healthspan, longevity.
Introduction
In a clinical setting, knowing a patient's biological age can be extremely useful. It offers a more accurate assessment of their health and disease risk, enabling personalised medical care. Patients with a biological age significantly higher than their chronological age may benefit from early interventions to mitigate age-related diseases. Additionally, biological age can help in tailoring lifestyle recommendations, such as diet and exercise, to improve overall health outcomes (Levine, 2013). Biological age, also known as physiological age, refers to how old a person seems biologically in contrast to their chronological age, which is the actual time a person has been alive. Unlike chronological age which simply counts the years, biological age considers various biomarkers to provide a more accurate representation of an individual's health, vitality, and functional capacity (Levine, 2013). Ageing clocks are also invaluable in research, particularly in the fields of gerontology and biogerontology. They allow scientists to quantify the effects of various interventions such as caloric restriction, exercise, and pharmacological treatments, on biological ageing. This capability accelerates the discovery of new anti-ageing therapies and enhances our understanding of the ageing process (Belsky et al. 2015). In the clinical setting, ageing clocks can be a useful tool when discussing medical and lifestyle interventions. It can be a great motivator for patients who have scored a biological age higher than their chronological age (Figure 1).
Figure 1: Knowing their biological age is great motivator for patients.
History of Ageing Clocks
The concept of biological age has evolved significantly over time, with the development of ageing clocks being a pivotal milestone. The history of ageing clocks began in earnest in the late 20th century, when scientists started to explore the molecular underpinnings of ageing. Early efforts were rudimentary, focusing on single biomarkers like telomere length and DNA damage levels (Harley et al., 1990).
In 2013, Steve Horvath developed the first widely recognized epigenetic clock, which uses DNA methylation levels to predict biological age with remarkable accuracy (Horvath, 2013). This innovation opened new avenues for understanding and measuring age at the molecular level. Examples of ageing clocks are listed in table 1.
The development of the biological ageing clock has evolved through several stages, leveraging various biological data types and machine learning techniques. Commercially, the most accessible ageing clocks are the epigenetic clocks and the haematological-based ageing clocks based on common blood biomarkers.
Table 1: Several types of ageing clocks have been developed since Horvath’s pioneering work.
|
Ageing clock |
Description |
Reference |
|
Epigenetic Clocks |
These clocks measure DNA methylation patterns to estimate biological age. Notable examples include the Horvath clock and the Hannum clock |
(Hannum et al. 2013) |
|
Transcriptomic Clocks |
These clocks analyse gene expression profiles. For instance, the RNA-based ageing clock uses blood samples to predict biological age. |
Peters et al. (2015) |
|
Proteomic Clocks |
These are based on the levels of proteins in the blood. Researchers have developed a proteomic clock that can provide insights into biological ageing by measuring the abundance of specific proteins. |
Johnson et al. (2020) |
|
Composite Clocks |
These integrate multiple types of biomarkers, including genetic, epigenetic, proteomic, and metabolomic data, to provide a comprehensive estimate of biological age. |
(Levine et al., 2018) |
|
Blood age clocks |
These clocks use blood biomarkers to predict a person’s biological age. Blood age clocks based on deep learning-based predictors were developed using anonymized blood test records from diverse populations. This innovative approach leverages machine learning algorithms to analyse complex biological data, offering a more nuanced understanding of ageing and overall health. |
(Zhavoronkov et al. 2019; Zhavoronkov and Mamoshina 2019) |
|
Face ageing clocks |
Face ageing clocks are advanced computational models used to estimate an individual's biological age based on facial features. These models typically employ machine learning and deep learning techniques to analyse various characteristics of the face, such as skin texture, wrinkles, and other age-related markers. |
(Wang et al. 2023; Yu et al. 2024) |
Epigenetic ageing clocks
DNA methylation patterns are used to predict chronological age. These patterns change predictably with age and can be measured accurately using high-throughput sequencing technologies. The first epigenetic ageing clock was developed by Hannum et al. in 2013, using methylation profiles from peripheral blood samples. It consisted of 71 CpG sites and demonstrated a root mean squared error of 4.9 years on independent data (Hannum et al. 2013). Steven Horvath further refined this approach in 2013 by developing a multi-tissue ageing clock using 353 CpG sites, achieving a median error of 3.6 years on the testing set. These clocks were developed using traditional machine learning approaches, such as linear regression with regularization (Horvath 2013). Several commercial epigenetic tests are now available for clinical use.
Ageing clocks based on deep neural network analysis
Routine blood test values, such as levels of glucose, cholesterol, and other biomarkers, are used to train deep learning models to predict age and health status. Early ageing clocks used traditional machine learning with linear regression and regularization to identify the most relevant features from a limited number of samples. More recent approaches use deep neural networks (DNN) to capture non-linear dependencies between input data and the target variable (age). These models can handle large datasets and complex relationships between features.
The first DNN-based ageing clocks were published in 2016 by Zhavoronkov's laboratory. These clocks were constructed using 41 blood test values from over 50,000 individuals, achieving a mean absolute accuracy of 5.5 years on previously unseen data (Bossi 2016). Subsequent work by Mamoshina et al. validated this approach on several million anonymised subject records from different populations, demonstrating the ability of DNNs to predict age and life expectancy based on blood parameters (Mamoshina et al. 2018). This work has culminated in the commercial development of the BloodAge analysis for clinical use.
Clinical applications of the ageing clocks
The clinical applications of the ageing clocks are diverse and impactful:
Predicting Health Trajectories: Ageing clocks can be used to predict an individual's health trajectory, including the onset of age-related diseases and overall life expectancy. This information can guide personalised healthcare interventions.
Evaluating Interventions: The clocks can assess the efficacy of various interventions, such as lifestyle changes, medications, and other therapies, by measuring their impact on biological age. For example, smokers are predicted to be older than their chronological age, and interventions to quit smoking can be monitored for their effectiveness in reducing biological age.
Personalised Medicine: Deep ageing clocks enable age-personalised medicine by tailoring treatments based on an individual's biological age rather than their chronological age. This
approach can optimize treatment outcomes and reduce adverse effects.
Insurance and Wellness Programs: Ageing clocks are being integrated into wellness programs and insurance models to incentivise healthy behaviours and manage health risks. For instance, programs that reward individuals for maintaining a younger biological age can promote healthier lifestyles and possibly reduce healthcare costs.
Haematological ageing clocks in clinical practice
Haematological ageing clocks aim to provide insights into an individual's biological age based on blood biomarkers. This innovative approach leverages machine learning algorithms to analyse complex biological data, offering a more nuanced understanding of ageing and overall health. The blood age analysis relies on advanced machine learning techniques to interpret a variety of blood-based biomarkers. The algorithm processes these inputs to generate an estimate of the biological age, which can then be compared to the individual's chronological age.
Blood biomarkers predict biological age
In the development of biological ageing clocks, chronological age plays a crucial role during the model training phase. The model is developed through four phases. During training and pattern recognition phases, the chronological age is used as the target variable to train the model, helping it learn the relationship between biomarkers and age. During the prediction phase the model predicts biological age based on biomarker levels without directly using the chronological age of the person being tested. The model finally evaluates the accuracy of the model assessed by comparing the predicted biological age to the chronological age (see table 2).
Table 2: The four phases of modelling.
|
Phases |
Tasks |
|
Training Data |
The training dataset includes both the blood biomarker levels and the chronological ages of the individuals. The chronological age serves as the target variable that the model aims to predict based on the biomarker data. |
|
Learning Patterns |
The machine learning model learns the relationships and patterns between the biomarkers and the chronological age. This involves identifying how changes in biomarker levels correlate with ageing. |
|
Prediction |
During the prediction phase, the model uses the learned relationships to estimate the biological age of new individuals based on their blood biomarker levels. The chronological age of the person being tested is not directly used in the prediction phase but is crucial for evaluating the accuracy of the model. |
|
Model Calibration |
The accuracy of the model is assessed by comparing the predicted biological age to the chronological age. The model is calibrated to minimize the error between the predicted biological age and the actual chronological age during the training process. |
The performance of the model is evaluated by comparing the predicted biological age to the actual chronological age. Metrics such as Mean Absolute Error (MAE) and R² (coefficient of determination) are used to assess how well the model's predictions align with the chronological ages in the validation dataset.
Blood biomarker studies
Mamoshina et al. 2018 conducted a big data study utilising deep learning-based predictors to develop haematological ageing clocks. The datasets included 20,699 blood test records from the Canadian population, 65,760 samples from the South Korean population, and 55,920 samples from the Eastern European population. Additionally, the study analysed the National Health and Nutrition Examination Survey (NHANES) dataset, which contained 55,751 samples with blood test values. The study found that the model trained on a combined dataset network achieved a coefficient of determination (R²) of 0.65 and a Mean Absolute Error (MAE) of 5.94 years. It concluded that ethnically diverse ageing clocks have the potential to predict chronological age and quantify biological age with greater accuracy than generic ageing clocks. The inclusion of population-specific data significantly improved the predictive accuracy of the models. The study identified several key biomarkers that were important for age prediction across different populations (see table 3).
The haematological ageing clocks developed in this study can be used to predict all-cause mortality and assess the biological age of patients. This can help in evaluating the effectiveness of healthspan-extending interventions and in personalised medicine (Mamoshina et al. 2018).
Table 3: Key biomarkers, when analysed in combination, that can reasonably accurately predict both chronological and biological age
|
Biomarker |
Significance |
|
Albumin |
The most prevalent protein in blood, crucial for regulating oncotic pressure. Deviations in albumin levels are associated with malnutrition, liver disease, chronic inflammation, and ageing. |
|
Glucose |
Blood glucose levels tend to increase with age and are associated with the ageing process through irreversible glycosylation of proteins |
|
Urea |
Levels of serum urea increase with age, linked to age-related decreases in muscle mass |
|
Haemoglobin |
Decreases in haemoglobin levels are common in the elderly and are associated with increased risks of cardiovascular disease, cognitive decline, and reduced quality of life |
|
Erythrocytes |
Important for oxygen transport, with variations affecting overall health and ageing |
|
Cholesterol (HDL-C and LDL-C) |
Levels of HDL- and LDL-cholesterol are important markers for cardiovascular health and ageing. |
|
Triglycerides |
Elevated levels are associated with metabolic syndrome and ageing |
BloodAge RESORT, BASE, and AMORIS studies
The RESORT study is a longitudinal, observational, prospective cohort study conducted at the Royal Melbourne Hospital in Melbourne, Victoria, Australia. The study investigated the association between biological age measured by a blood biochemistry BloodAge clock and frailty in geriatric rehabilitation inpatients. The study included 1,187 patients with a median age of 83.4 years, of which 57.4% were female. Data collected included age, sex, ethnicity, length of stay in acute hospitalization and rehabilitation, primary reasons for acute admission, comorbid conditions evaluated by the Cumulative Illness Rating Scale (CIRS), cognitive impairment, medication count, malnutrition risk, physical performance, and functional performance (Guan et al. 2024).
The study found that the blood biomarker biological age was strongly correlated with chronological age (Spearman r = 0.883). Furthermore, higher biological age was associated with more severe frailty at admission (OR: 1.053, 95% CI: 1.012–1.096) in patients with a CIRS score of <12, but not in patients with a CIRS score >12. Lastly, the study found that a higher biological age was associated with frailty in cardiac, haematological, respiratory, renal, and endocrine domains. Higher biological age, determined by the blood-based ageing clock, was associated with severe frailty at admission (Guan et al. 2024). The study demonstrated that the blood age clock could be used to identify patients at higher risk of severe frailty, particularly those with fewer comorbid conditions. This information can be valuable for tailoring rehabilitation programs and monitoring the effectiveness of interventions in geriatric patients (Guan et al. 2024), see table 4.
Table 4: Benefits of blood age testing in geriatric patients
|
Benefit of blood age testing |
Outcome |
|
Identify High-Risk Patients |
By identifying patients at higher risk of severe frailty, healthcare providers can tailor rehabilitation programs to address specific needs. |
|
Monitor Intervention Effectiveness |
The clock can be used to monitor the effectiveness of various interventions, such as physiotherapy and nutritional therapy, by measuring their impact on biological age. |
|
Personalise Treatment Plans |
The clock enables age-personalised medicine by tailoring treatments based on an individual's biological age rather than their chronological age, optimizing treatment outcomes and reducing adverse effects |
The Berlin Ageing Study (BASE) aimed to define biological age using standard laboratory blood tests and to examine its association with morbidity and mortality, independent of chronological age. The study found that older biological age was associated with higher morbidity and mortality hazards, even after accounting for chronological age, sex, and education.
The study utilised data from two cohorts: The Berlin Aging Study (BASE) which included 384 participants aged 70-102 years, with a mean age of 84.35 years, and the Berlin Aging Study II (BASE-II) which included 1,517 participants aged 60-85 years with a mean age of 68.66 years. Half the participants in each cohort were women. Twelve standard laboratory biomarkers reflecting metabolic, cardiovascular, inflammatory, and kidney functioning were selected based on their association with mortality in the BASE cohort. The BASE Cohort study found that older biological age was associated with higher physician-observed morbidity and mortality hazards, independent of chronological age, sex, and education. The BASE-II Cohort study found that older biological age was associated with higher physician-observed morbidity and lower subjective health, but not lung capacity. These associations remained significant even when considering alternative biomarkers like telomere length and DNA methylation age (Drewelies et al. 2022). The biological age composite based on standard blood tests is cost-effective, non-invasive, and easy to implement in clinical settings. It offers a practical approach for assessing the biological age of older adults, which can help in identifying individuals at higher risk of morbidity and mortality. Blood biomarker tests can be used to guide interventions aimed at improving health outcomes and extending healthy lifespan.
The Swedish AMORIS cohort study aimed to investigate the differences in blood biomarker profiles between individuals who lived to be 100 years old (centenarians) and those who did not. The study also explored the association between specific biomarker values and the likelihood of reaching age 100, as well as the homogeneity of biomarker profiles among centenarians. Twelve blood-based biomarkers related to inflammation, metabolic, liver, and kidney function, as well as potential malnutrition and anaemia, were included. In total, 1224
participants (84.6% females) lived to their 100th birthday.
Centenarians displayed more favourable biomarker values from age 65 onwards compared to non-centenarians. Higher levels of total cholesterol and iron, and lower levels of glucose, creatinine, uric acid, aspartate aminotransferase (ASAT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LD), and total iron-binding capacity (TIBC) were associated with a greater likelihood of becoming a centenarian.
The logistic regression analysis showed that all included biomarkers except for alanine aminotransferase (ALAT) and albumin were predictive of the likelihood of reaching age 100. A dose-response relationship was found for uric acid, with individuals in the lowest quintile having almost twice the chance of reaching age 100 compared to those in the highest quintile.
In conclusion, the study found that from age 65 onwards, individuals who eventually became centenarians displayed more favourable biomarker values than those who did not. The study highlights the potential role of genetic and lifestyle factors in achieving exceptional longevity and emphasizes the importance of specific biomarker characteristics in longevity research (Murata et al. 2024)
Accuracy and Validation
The accuracy of the haematological ageing clock known as BloodAge analysis has been validated through extensive research and clinical trials. The model has been trained on large datasets encompassing diverse populations, which enhances its robustness. Peer-reviewed studies have corroborated the tool's predictive power, demonstrating that it can reliably estimate biological age and correlate it with health outcomes (Putin et al. 2016). The BloodAge analysis has significant utility in research, particularly in the fields of gerontology and precision medicine. By providing a quantifiable measure of biological age, researchers can better understand the impact of various interventions, such as lifestyle changes or pharmaceutical treatments, on the ageing process. This tool can also help identify biomarkers associated with accelerated ageing, thereby uncovering potential targets for anti-ageing therapies (Zhavoronkov et al. 2019; Zhavoronkov and Mamoshina 2019). The blood biomarkers utilised by the BloodAge Analysis are listed in figure 2.
Clinical Applications of BloodAge analysis
Biological age testing can predict susceptibility to age-related diseases before symptoms appear, allowing for early intervention and prevention. Understanding the biological age of your patients enables you to tailor their health and wellness strategies, including diet, exercise, and sleep habits, to improve your overall health. As the saying goes, “If you can measure it, you can improve it". The report graphically displays which biomarkers are contributing to a higher biological age, and those that are associated with a lower biological age (see figure 3). In a clinical setting, the BloodAge analysis offers several benefits as listed in table 5.
Table 5: Clinical benefits of BloodAge analysis.
|
Application in clinical practice |
Benefits |
References |
|
Personalised Medicine |
By knowing a patient's biological age, healthcare providers can tailor treatments and preventive measures more effectively. For example, individuals with a higher biological age may require more aggressive interventions to mitigate age-related diseases. |
(Mamoshina et al. 2018; Zhavoronkov et al. 2019; Zhavoronkov and Mamoshina 2019) |
|
Risk Assessment |
The tool can help in assessing the risk of various age-related conditions, such as cardiovascular diseases, diabetes, and neurodegenerative disorders. This enables early detection and timely intervention. |
(Mamoshina et al. 2018; Zhavoronkov et al. 2019; Zhavoronkov and Mamoshina 2019) |
|
Monitoring Health Over Time |
Patients can use the blood age analysis to monitor their biological age over time, providing feedback on the effectiveness of lifestyle changes or medical treatments. This ongoing monitoring can motivate individuals to adhere to healthier habits. |
(Zhavoronkov et al. 2019; Zhavoronkov and Mamoshina 2019) |
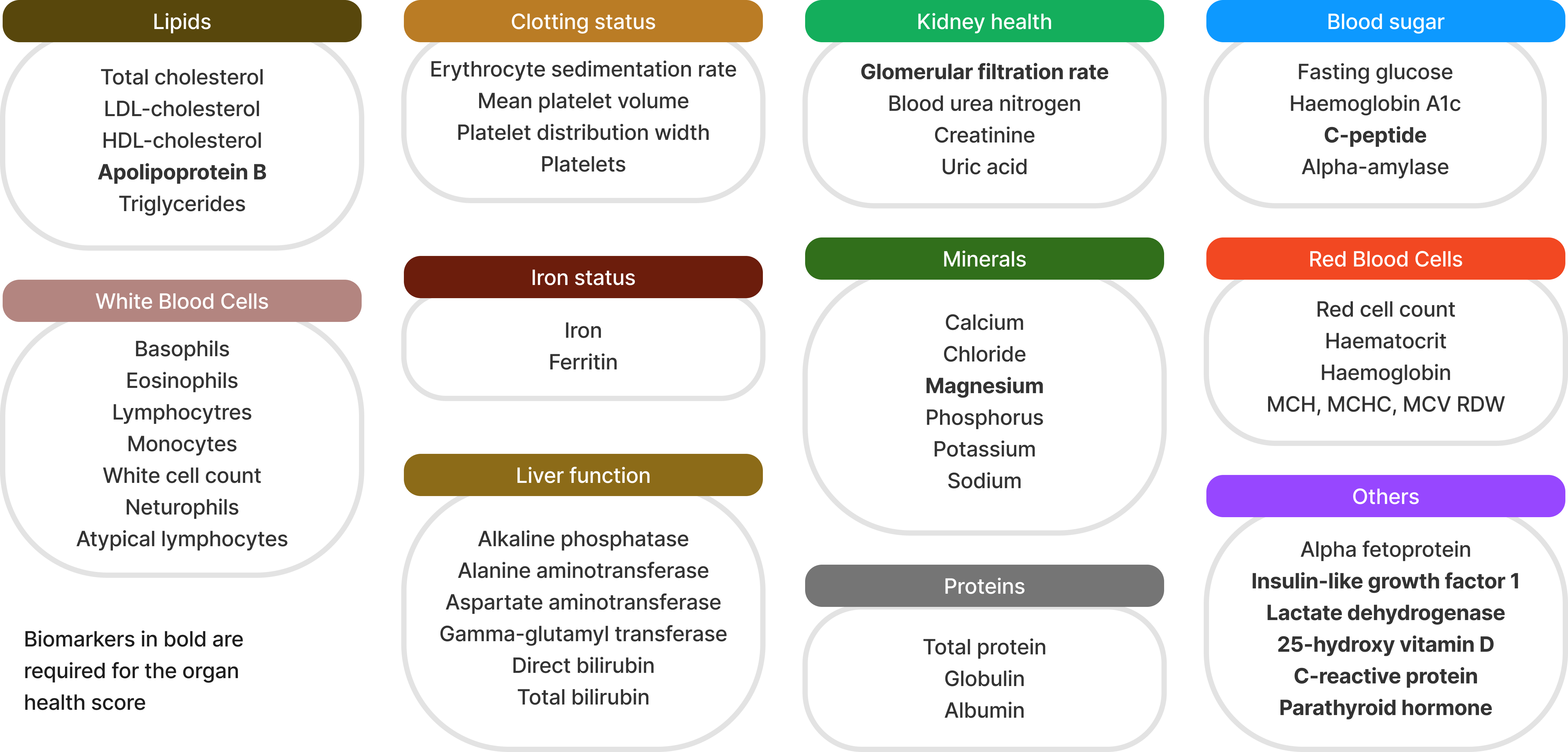
Figure 2: Biomarkers used in the BloodAge analysis
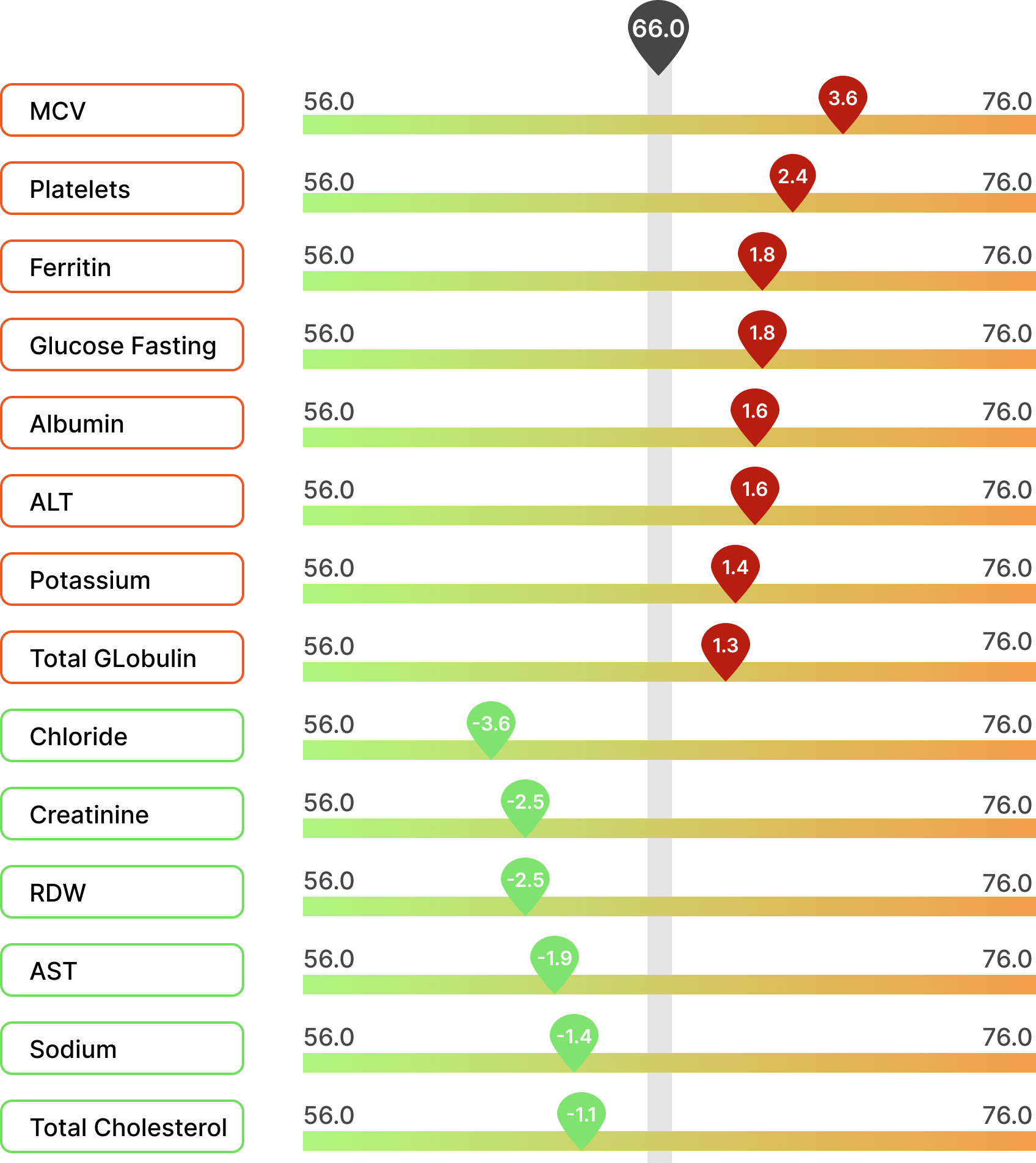
Figure 3: The BloodAge analysis provides feedback on which biomarker contributes the most to the increased and decreased biological age.
The Future of Ageing Clocks
The future of ageing clocks looks promising, with ongoing research aiming to refine their accuracy and expand their applicability. Advances in machine learning and artificial intelligence are likely to enhance the predictive power of these clocks. Moreover, as more longitudinal data becomes available, ageing clocks will become increasingly personalised, offering insights tailored to individual health profiles (Fahy et al. 2019).
Bibliography
Belsky, D. W., A. Caspi, R. Houts, H. J. Cohen, D. L. Corcoran, A. Danese, H. Harrington, S. Israel, M. E. Levine, J. D. Schaefer, K. Sugden, B. Williams, A. I. Yashin, R. Poulton, and T. E. Moffitt. 2015. 'Quantification of biological aging in young adults', Proc Natl Acad Sci U S A, 112: E4104-10.
Bossi, G. 2016. 'MKK3 as oncotarget', Aging (Albany NY), 8: 1-2.
Drewelies, J., G. Hueluer, S. Duezel, V. M. Vetter, G. Pawelec, E. Steinhagen-Thiessen, G. G. Wagner, U. Lindenberger, C. M. Lill, L. Bertram, D. Gerstorf, and I. Demuth. 2022. 'Using blood test parameters to define biological age among older adults: association with morbidity and mortality independent of chronological age validated in two separate birth cohorts', Geroscience, 44: 2685-99.
Fahy, G. M., R. T. Brooke, J. P. Watson, Z. Good, S. S. Vasanawala, H. Maecker, M. D. Leipold, D. T. S. Lin, M. S. Kobor, and S. Horvath. 2019. 'Reversal of epigenetic aging and immunosenescent trends in humans', Aging Cell, 18: e13028.
Guan, L., C. S. L. Tuttle, F. Galkin, A. Zhavoronkov, and A. B. Maier. 2024. 'Higher blood biochemistry-based biological age developed by advanced deep learning techniques is associated with frailty in geriatric rehabilitation inpatients: RESORT', Exp Gerontol, 190: 112421.
Hannum, G., J. Guinney, L. Zhao, L. Zhang, G. Hughes, S. Sadda, B. Klotzle, M. Bibikova, J. B. Fan, Y. Gao, R. Deconde, M. Chen, I. Rajapakse, S. Friend, T. Ideker, and K. Zhang. 2013. 'Genome-wide methylation profiles reveal quantitative views of human aging rates', Mol Cell, 49: 359-67.
Horvath, S. 2013. 'DNA methylation age of human tissues and cell types', Genome Biol, 14: R115.
Mamoshina, P., K. Kochetov, E. Putin, F. Cortese, A. Aliper, W. S. Lee, S. M. Ahn, L. Uhn, N. Skjodt, O. Kovalchuk, M. Scheibye-Knudsen, and A. Zhavoronkov. 2018. 'Population Specific Biomarkers of Human Aging: A Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations', J Gerontol A Biol Sci Med Sci, 73: 1482-90.
Murata, S., M. Ebeling, A. C. Meyer, K. Schmidt-Mende, N. Hammar, and K. Modig. 2024. 'Blood biomarker profiles and exceptional longevity: comparison of centenarians and non-centenarians in a 35-year follow-up of the Swedish AMORIS cohort', Geroscience, 46: 1693-702.
Putin, E., P. Mamoshina, A. Aliper, M. Korzinkin, A. Moskalev, A. Kolosov, A. Ostrovskiy, C. Cantor, J. Vijg, and A. Zhavoronkov. 2016. 'Deep biomarkers of human aging: Application of deep neural networks to biomarker development', Aging (Albany NY), 8: 1021-33.
Wang, Yiyang, Kehang Mao, Haotian Zhai, and Jing-Dong Jackie Han. 2023. 'Clinical application of facial aging clocks', The Lancet Regional Health – Western Pacific, 37.
Yu, Z., Y. Zhou, K. Mao, B. Pang, K. Wang, T. Jin, H. Zheng, H. Zhai, Y. Wang, X. Xu, H. Liu, Y. Wang, and J. J. Han. 2024. 'Thermal facial image analyses reveal quantitative hallmarks of aging and metabolic diseases', Cell Metab, 36: 1482-93 e7.
Zhavoronkov, A., R. Li, C. Ma, and P. Mamoshina. 2019. 'Deep biomarkers of aging and longevity: from research to applications', Aging (Albany NY), 11: 10771-80.
Zhavoronkov, A., and P. Mamoshina. 2019. 'Deep Aging Clocks: The Emergence of AI-Based Biomarkers of Aging and Longevity', Trends Pharmacol Sci, 40: 546-49.